External Morphology
Valves
The most distinction and diagnostic feature of a polyplacophoran is their eight shell valves. The morphology of these valves are useful to distinguish between taxa, with the tegmentum found to be the most useful characteristic to distinguish between some Acanthochitona species (Bonfitto et al 2011). Using scanning electron microscopy (SEM), Acanthochitona sp. values will be used to further describe this species.
Methods:
On Heron Island, the valves and radula were obtained from Acanthochitona sp. using techniques outlined in Andrade and Solferini 2006 and suggested by Douglas Eernisse, a professor at the California State University, Fullerton.
Acanthochitona sp. was relaxed in the freezer for 15 minutes than a foot sample was obtained and stored in 70% ethanol for DNA. In a small glass petri dish, a monolayer of potassium hydroxide was added along with cool water and the chiton. This was placed under a fume hood on a heating plate set at 50ºC and brought to near boil to disarticulate and clean the valves and girdle hard parts. This was left until flesh had disarticulated from around the plates and the radula. The radula and plates were then rinsed in fresh water and stored in centrifuge tubes with water to bring back to the university.
Back at the University of Queensland, the samples were dehydrated in a series of ethanols (50%, 70%, 90% and 100% x 2) using the Biowave (Pelco) microwave that was fitted with a steady temperature unit to keep it operating at room temperature. The samples were then air-dried and placed on double-sided carbon tabs on stubs. The stubs were coated with gold using a SPI sputter coater and samples were imaged using a Jeol Neoscope 5000 tabletop scanning electron microscope at an acceleration voltage of 10kV. Below is the description of Acanthochitona sp. valves. For a description of the radula, please see (Internal anatomy-link!).
Head valve:
The head valve of Acanthochitona sp. is semi-circle in shape and from the dorsal view consists mostly of the tegmentum, which is the part of the valve that is exposed. The articulamentum is imbedded in the mantle but is easily distinguishable from tegmentum on Acanthochitona sp. valves as it is porcelain white and smooth. Some of the articulamentum projects past the reduced tegmentum that is common in many species in this genus but this is hard to distinguish from the image provided (Schwabe 2010)(Figure 1A). On the ventral side of the head valve, the anterior margin demonstrates 5 slits on the projection of the articulamentum known as the insertion plate, which is characteristic of the genus (Lyons 1988, Jones and Baxter 1987) (Figure 1B). In-between these slits are six smooth teeth that face anteriorly. The insertion plates holds the valves in place as they project into the mantle (Ruppert et al 2004). The posterior margin of the head valve is nearly straight, with an apex in the middle.
Intermediate valves
The morphology of the intermediate valves of Acanthochitona sp. are all very similar. Insertion plates occur laterally of the tegmentum and one slit is present which is common in many Acanthochitona species (Jones and Baxter 1987, Lyons 1988)(Figure 1C/D). The two anterior enlargements of the articulamentum are known as the sutural laminae, which are smooth and pointed, and seperated by a flat jugal sinus (Figure 1C/D). The sutural laminae is present on all valves except for the head valve. The sutural laminae connects the valves together as they sit beneath the posterior margin of the preceeding valve and are connected my muscular tissue (Jones and Gowlett-Holmes 1998). The tegmentum is divided into two areas, one is a triangular area known as the central jugal area that is wide at the anterior and decreases towards the posterior apex (Figure 1C)(Figure 2B/C). The other area of the tegmentum is known as the pleurolateral area (Schwabe 2010).
Tail valve
The tail valve is very useful to differentiate between different polyplacophoran taxa (Scwabe 2010). Acanthochitona sp. tail valve has a posterior insertion plate that is rounded and smooth with two slits on the edges. The sutural laminae occur laterally of the tegmentum and have a parallelogram ("squashed rectangle") shape and are separated by a rounded, smooth jugal sinus (Figure 1F). The tegmentum of the tail valve is round and has a point known as the mucro, just behind the centre (Figure 1E). The area before the mucro is known as the antemucronal area and the area after the mucro is known as the postmucronal area, however it is not very distinct in Acanthochitona sp. The postmucronal area also has a jugal area like the intermediate valves but it is not very distinct in Acanthochitona sp. After the mucro is the postmucral slope which can be seen from a side view and Acanthochitona sp. slope is shallow and dome-shaped.
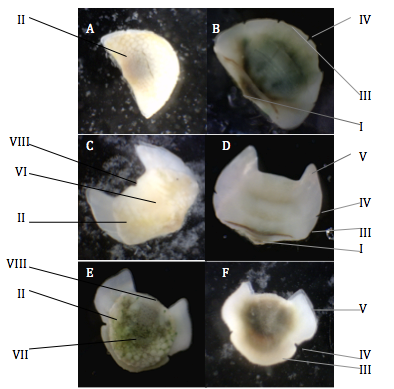
Figure 1: Acanthochitona sp. valves under a light microscope. A: Head valve (dorsal view). B: Head valve (ventral view). C: 5th valve(4th intermediate valve) (dorsal view). D: 5th valve(4th intermediate valve) (ventral view). E: Tail valve (dorsal view). F: Tail valve (ventral view). I: Apex. II: Tegmentum. III: Insertion plates (teeth). IV: Slits. V: Sutural laminae. VI: Jugal area. VII: Mucro. VIII: Jugal sinus. Terminology from Jones and Gowlett-Holmes 1998 and Schwabe 2010.
Polyplacophorans valves do not just consist of the tegmentum and articulamentum, they are four layers which make up each valve. The tegmentum and articulamentum are the only two layers that are used to distinguish between species (Schwabe 2010). The periostracum, is the first layer that is thin, organic and proteinaceous and easily erodes away to expose the tegmentum. The second layer is the tegmentum, which is made up of conchoilin (proteins excreted by the mantle) and calcium carbonate. The third and thickest layer is the articulamentum that is made up of argonite which are calcium carbonate crystals that are arranged in a crossed-lamellar pattern to create strength. The fourth layer that lines the ventral surface of the value is the hypostracum which is made up of a thin layer of crystals (Kaas et al. 1998, Ruppert et al 2004).
Tegmentum sculpture
All Acanthichitona species tegmentum is characterised as having round or drop shaped granules that are usually elevated (Bonfitto et al 2011). Acanthochitona sp. head valve is covered in irregular raised pustolose granules that are well rounded to slightly elongate in shape (Figure 2A). The granules become more pronounced and increase in size and elevation towards the anterior end of the valve. These granules also occur on the pleurolateral area of the intermediate valves and are well rounded but not as elevated compared to the head valve granules (Figure 2B/C). They are almost longitudinally positioned, coming off the jugal area. On the tail valve, rounded, raised granules occur in the postmucronal area of the tegmentum only. The SEM image (Figure 2D) does not demonstrate this, as the tegmentum of the tail valve appears to be damaged from the drying process but this can be seen in the light microscope image (Figure 1E). The granules that occur on the head valve, pleurolateral area and postmucronal area are collectively know as radial structures (Schwabe 2010). The structures that occur in the jugal area of the valves are known as longitudinal structures (Schwabe 2010). The jugal area of Acanthochitona sp. intermediate valves and antemucronal area of the tail valve are smooth, with no particular sculpture associated with them (Figure 2B/C). There is a gradual transition from the smooth jugal area to the circular granules on the intermediate valves. There are many small, pore-like structures on the surface tegmentum, these are known as aesthetes.
Figure 2: Scanning electron microscope images of the dorsal side of Acanthochitona sp. valves. Valves are arranged with the anterior margin to the left and the posterior margin to the right. A: Head valve. B: 3rd valve (2nd intermediate valve). C: 5th valve (4th intermediate valve). D: Tail valve. Note: articulamentum disintegrated during the drying process, refer to Figure 1 for articulamentum morphology.
Aesthetes
Aesthetes are the shell organs that occur on the valve tegmentum and are unique to polyplacophorans (Ruppert et al 2004). They consist of pores on the tegmentum which open into a canal system filled with epidermal cells and tissue that have been proposed to be sensory, excretory, mechanoreceptors and chemoreceptors but their actual functions is still unclear and disputed (Kaas et al. 1998). All polyplacophorans have aesthete organs, but two other shell organs are known for their photoreceptive ability, the intrapigmentary ocellus and the extrapigmentary ocellu. These only occur in the suborder Ischnochitonia, and thus Acanthochitona sp. like others in the Acanthochitonidae family do not possess ocelli (Gowlette-Holmes 2001, Kass et al 1998). Aesthetes consist of two parts that occur in bundles, the megalethetes is the central epidermal lined canal with many cell types in the tegmentum and these have numerous smaller unicellular canals that branch off it, known as micresthetes (Schwabe 2010). They both end in flat caps at the surface of the tegmentum, megalethetes end in an apical cap and micresthetes end in a supsidiary cap (Kaas et al 1998). Once valves are cleaned, aesthetes can be differentiated as the megalethetes ends with a large pore on the tegmentum, whereas the micresthetes have smaller pores (Schwabe 2010).
Acanthochitona sp. aesthetes occur on the pustolose granules of the head valve, intermediate valves and tail valve and are also on the jugal area of the intermediate and tail valves (Figure 2A,B,C,D). Like other Acanthochitona species that have a pustolous granulated tegmentum, the megalesthetes occur on the granule only, while the micresthetes occur on and between the granules (Kaas 1998). This is seen on Acanthochitona sp. head valve (Figure 3A) and also on the pleurolateral areas of the intermediate valves and postmucroal area of the tail valve. Each granule, has about four megalesthetes each in the middle (Figure 3A), usually in a square shape but this is variable. The megalesthetes are surrounded by a highly variable number of micresthetes which appear to be uniformly distributed. Acanthochitona critinita in comparison to Acanthochitona sp. has much flatter and drop shaped granules, with more megelaesthetes located slightly posteriorly on the granule (Figure 3D). Although not demonstrated in the image, Acanthochitona critinita megelaesthetes are surrounded by 12-16+ micresthetes that only appear to occur on the granule, demonstrating the variability in tegmentum structure within one genus.
On the intermediate valves, the aesthetes are most prominent and larger on the jugal area of the tegmentum (Figure 3B) compared to aesthetes on the granules. The most prominent aesthetes are found on the centre to the posterior side of the valve, on the jugal area (Figure 2B and C). The megalesthetes appear to be irregularly distributed and surrounded by a large number of micresthetes (Figure 3B).
Figure 3: Scanning electron microscope images of Acanthochitona sp. (A,B,C) and Acanthochitona crinita (D) aesthethes on the valve tegmentum. A: Close up of the anterior area of the head valve. B: Close up of jugal area of the 3rd valve. C: Close up of micresthetes on the jugal area of the 3rd valve. D: Close up of granules of Acanthochitona crinita. Image D from Bonfitto et al. 2011.
Note: For a description on the rest of the external morphology, see Description and to learn about Acanthochitona sp. gills, see Respiration.
|